The white shrimp (Litopenaeus vannamei) is one of the most widely farmed crustacean species in global aquaculture. In recent years, large-scale intensive farming has become the dominant trend. However, the environmental stress associated with intensive farming has led to frequent disease outbreaks. Before discussing how to manage these diseases, it is essential to understand the immune system of Vannamei shrimp.
I. Basic Definition of the Immune System
The immune system is a key biological mechanism that protects organisms from foreign pathogens. In vertebrates (such as fish), the immune system is mainly divided into two types: innate immunity and adaptive immunity, which work together to maintain the organism's health.
- The innate immune system, also known as nonspecific immunity, is the first line of defense present from birth. It provides a rapid and broad defense, including mechanisms such as phagocytic cells that can quickly recognize and eliminate invading pathogens.
- The adaptive immune system (AIS), also known as acquired immunity, develops gradually after exposure to specific pathogens. It is primarily mediated by T cells and B cells, offering high specificity and the ability to mount a stronger and faster response upon re-exposure to the same pathogen.
However, the immune system of shrimp differs from that of fish. Shrimp do not have the AIS and rely entirely on nonspecific immunity to combat pathogens. Due to the lack of immune memory, white shrimp have limited resistance to various infections, making large-scale shrimp farming more challenging than fish farming.
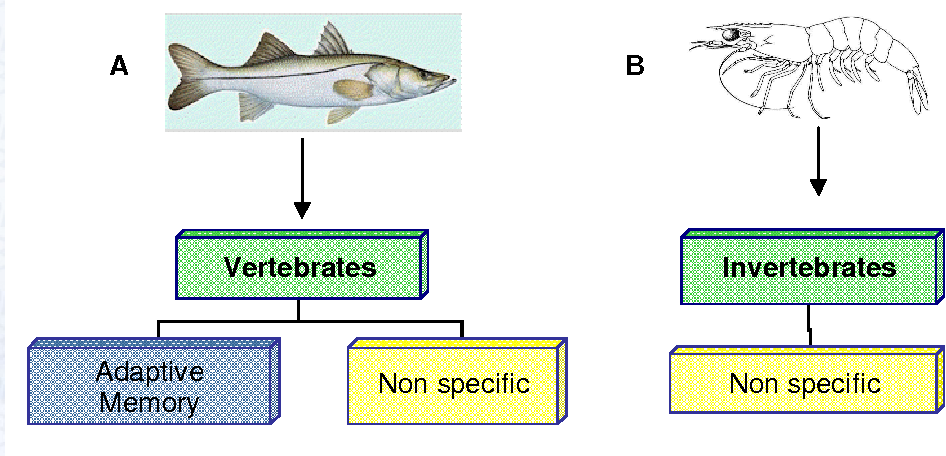
A comparison of the immune systems in fish and shrimp. (Martínez, 2011)
II. Three Main Lines of Innate Immunity
The innate immune system of white shrimp can be divided into three main lines of defense: physical and chemical barriers, humoral immune responses, and cellular immune responses.
-
1. Physical and Chemical Barriers:
The shrimp’s exoskeleton not only protects against physical damage but also serves as the first line of defense against invading pathogens. In addition to this hard outer shell, mucosal tissues such as the epidermis and intestinal lining also play an important role in defense. These tissues act as physical shields to prevent pathogens from entering the shrimp body.
Furthermore, antimicrobial peptides (AMPs) are frequently found in mucosal tissues and surrounding hemolymph. These are small protein molecules, primarily secreted by hemocytes, that can directly disrupt the cell membranes of pathogens—functioning like the shrimp's own natural antibiotics. Once released, AMPs diffuse through mucosal areas and the hemolymph to exert their antimicrobial effects and help prevent further pathogen infection.
Although some studies suggest that specific mucosal tissues may also express AMPs, their primary source remains the immune cells found in the blood.

Shrimp exoskeleton. (Image generated by AI)
If the physical defense fails to prevent pathogens from infecting, the shrimp will activate its humoral and cellular immune responses to suppress further infection.
2. Humoral Immunity
Once pathogens invade, various enzymes and immune effectors in the shrimp are activated. The key components involved include:
- Antimicrobial Peptides (AMPs):
AMPs are one of the most crucial defensive factors in the humoral immunity of white shrimp. They are often the earliest system to be activated after pathogen invasion, capable of rapidly inhibiting pathogens.
White shrimp express multiple AMPs, including Penaeidins, and Crustins. These peptides possess broad-spectrum antimicrobial activity, able to disrupt bacterial membranes. Additionally, certain AMPs can bind to viral envelope proteins, suppressing viral entry or replication. However, current research mainly focuses on their direct effects against bacteria and fungi. These AMPs are synthesized primarily by hemocytes and function by diffusing into the hemolymph after release.
Lysozyme plays a key role in defending against bacterial infections. It is an enzyme that hydrolyzes peptidoglycan in bacterial cell walls, offering broad-spectrum antimicrobial activity. In addition to its strong action against bacteria, lysozyme may also help prevent secondary bacterial infections during viral challenges by controlling opportunistic bacterial proliferation.
- Prophenoloxidase System (proPO system):
Due to the shrimp’s open circulatory system—which lacks closed blood vessels and lymphatic barriers—pathogens that enter the body can easily spread. Therefore, the proPO system plays a vital role in restricting their movement.
The proPO system is the core component of humoral immunity in white shrimp. When immune-related proteins or receptors in the hemolymph recognize invading pathogens, they activate proPO, which is then converted into active PO. This triggers local clotting and melanization reactions, leading to melanin deposition at the site of infection. This process helps encapsulate and isolate pathogens, preventing their spread, while producing reactive oxygen species (ROS) and free radicals to eliminate them. It also contributes to wound healing.
The shrimp’s humoral immunity also includes a well-developed antioxidant defense system, involving enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). These systems help eliminate ROS generated during immune responses or by pathogens themselves. This mechanism also clears ROS byproducts from the proPO pathway, preventing self-damage and maintaining a balance between immune activation and cellular health.
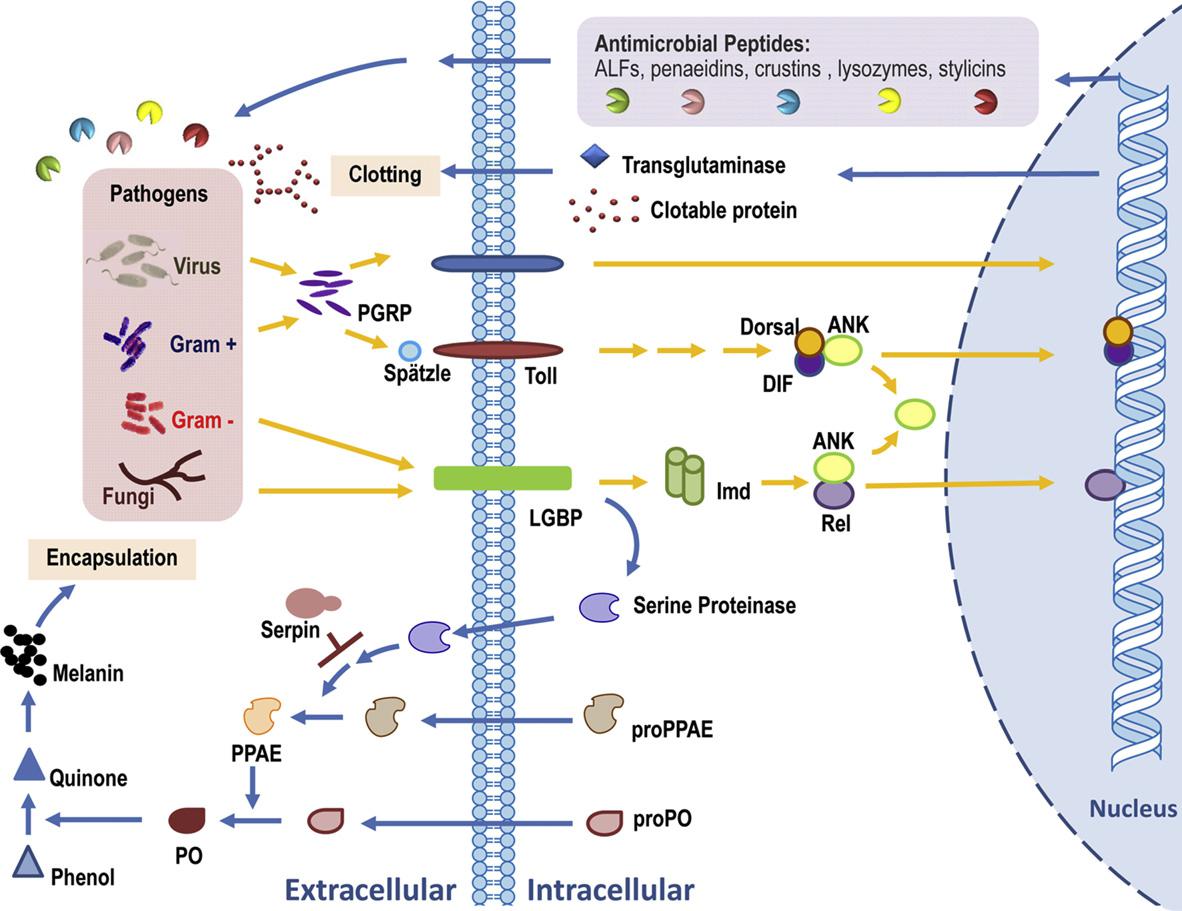
A schematic model of the shrimp immune system (Tassanakajon et al., 2013.)
3. Cellular Immunity:
Hemocytes in shrimp are classified into three types, each responsible for different cellular immune tasks:
Small in size, are typically the first immune cells to respond upon pathogen invasion. They make up about 10–20% of the total hemocyte population.
- Semi-granular cells (SGCs):
Contain a small number of cytoplasmic granules and are the most abundant hemocyte type, comprising 60–75% of total hemocytes. They serve as the primary effectors in cellular immune responses.
The largest hemocytes, filled with numerous granules. They constitute about 10–20% of hemocytes and are slower to activate, but play key roles in fighting large pathogens and sustained infections.
Shrimp hemocytes are capable of executing numerous immune functions, including phagocytosis, release of AMPs, and initiating respiratory burst reactions that generate reactive oxygen species (ROS) to attack pathogens.
They can also participate in encapsulation and nodule formation to surround and eliminate larger pathogens such as parasites. Additionally, some hemocytes may undergo apoptosis upon detecting viral infection, thereby limiting viral replication and spread within host cells.
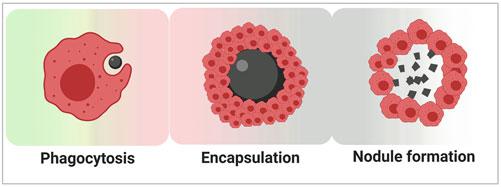
Diagrammatic representation of phagocytosis, encapsulation and nodule formation. (Rajendran et al., 2022)
Cellular and humoral immune responses in shrimp are not independent; rather, they are closely interconnected. For example, activation of the proPO system is triggered by enzymes released from SGCs. Once the system is activated, GCs become further participate in phagocytic activity at the infection site. Moreover, the production and release of AMPs—one of the central components of humoral immunity—are carried out by both SGCs and GCs.
This well-coordinated interaction between cellular and humoral components highlights how, even without adaptive immunity, white shrimp can construct an effective multi-layered immune defense through the orchestration of innate immune mechanisms.
Reference:
- THE IMMUNE SYSTEM OF SHRIMP. - Martínez, F.S, 2011.
- Discovery of immune molecules and their crucial functions in shrimp immunity. - Tassanakajon et al., 2013.
- The prophenoloxidase-activating system in invertebrates. - Cerenius & Söderhäll, 2004.
- Shrimp Immune System and Immune Responses. - Rajendran et al., 2022.




